eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.
The technical characteristics and unique formula make eSwab® an extremely versatile device that can optimize your laboratory workflow, and reduce costs.
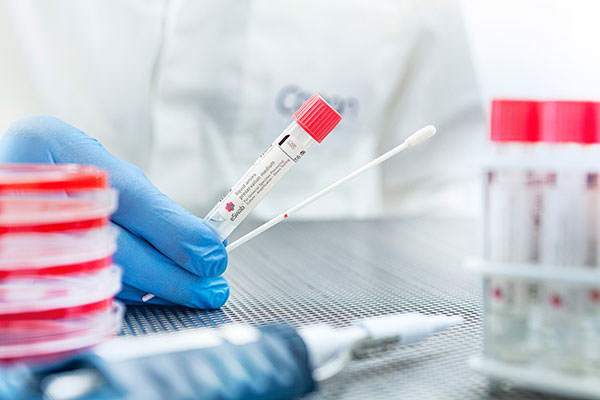
Not only eSwab®- collected samples are compatible with WASP®-automated processing: eSwab® is the product that has opened the door to the concept of Liquid Based Microbiology, the first step into the Full microbiology Lab Automation.

eSwab® allows for multipurpose testing, traditional and automated bacterial culture, Gram stain, NAAT, and rapid antigen assays.

Ensure a quick, capillarity-driven sample uptake and a
superior elution of the biological specimen, expanding
downstream diagnostic testing capabilities.


eSwab® is compliant with CLSI M40-A2 Quality Control
for Microbiological Transport System standards.
Thanks to its liquid formulation, it could be used to run multiple tests from a single specimen, reducing multiple sampling and stocking costs.

eSwab® is available combined with single or multiple swabs to meet different laboratories’ needs.

eSwab® preserved the viability of all the organisms tested for 48 hours at both controlled room and refrigerated temperature, except for Neisseria gonorrhoeae cultures which should be processed within 24 hours. It can be used for the preservation of bacterial, viral or Chlamydial antigens and nucleic acids from swab spedimens for five days when stored at room temperature, 7 days if stored at 4°C and up to 6 months when stored at -20° C.
The combination of with FLOQSwabs® expands your possibilities even further by ensuring an unmatched specimen collection in many anatomical collection sites. Discover why we call them “the perfect collection device.”

This product line groups a variety of product codes, each optimized to address specific needs.
Check here the summary of these features and contact us to discover the product code that best suits your need-mix and available in your Country.
Always faithful to our scientific approach, we are eager to hear the proof of the success of our efforts and commitment.








For aereobe, anaerobe, and fastidious bacteria detection

eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.
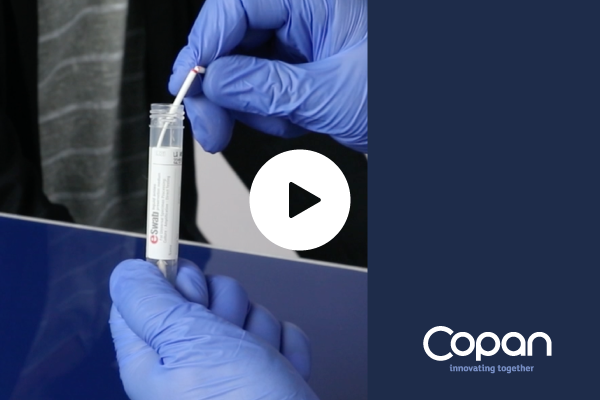
eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.

eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.

eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.

eSwab® – Copan’s Liquid Amies Elution Swab – collection and transport system is our multipurpose media intended for the collection and transport of clinical specimens containing aerobes, anaerobes, fastidious bacteria, viruses and Chlamydia.


Write us to satisfy your curiosity, get information or start a great collaboration!
Use our search tool and be surprised by how easy it is to get right to the point.